In a small laboratory, not far from southern California’s Pacific coastline, Dmitri Lapotko is using lasers to conduct on-demand explosions on a scale almost infinitely small. These explosions are carefully designed to obliterate cancer cells at a nanoscale, with a level of efficiency and safety which far outmatches the current treatments of choice. The technology, pioneered by the company Masimo, is about to undergo clinical trials for both the diagnosis and treatment of cancer in the next few years. But the story of how the idea was first conceived originates from one of most defining moments of the 20th century.
In the late 1980s, Lapotko was a laser weapons physicist for the Soviet Union, living and working in what is now Belarus. His particular expertise was in using airborn ultrasound to steer the laser beam of a weapon in the upper atmosphere, as the Soviets tried to match the threat of Ronald Reagan’s Strategic Defense Initiative, nicknamed ‘Star Wars.’
But with the end of the Berlin Wall and the subsequent disintegration of the Soviet Union, many weapons scientists found themselves left out in the cold, surplus to requirements and with few career prospects.
“This was a bitter time for many Soviet physicists,” Lapotko remembers. “We realised our work was not about science or the future, but politics.”
However just as many of the scientists involved in the Manhattan Project 40 years earlier subsequently turned to biomedical research, Lapotko decided to try and apply his knowledge of lasers to treat diseases at the cell level, and the biggest challenge of all, developing a novel means of detecting and treating cancer, initially in Belarus and then in the US.
“One of the biggest problems in cancer treatment is that we cannot detect micro tumours at the earliest stage and we often would not be able to remove them surgically without damaging nearby important cells and organs,” Lapotko says. “Currently, the minimal detectable tumours are already several millimetres big and by then the disease has developed.”
Chemotherapy and radiation therapy are not always effective because cancer cells continuously mutate and so rapidly develop resistance, requiring therapeutic doses which harm the patient in order to destroy them. “You can have an excellent drug today, but tomorrow it doesn’t work,” Lapotko says. “So I decided to base my approach on a way to detect and explode the cancer cell mechanically, something it cannot resist through its biological tricks. If you do this, there’s no biological way it can reassemble, revive or metastasise.”
Over the past two decades, researchers have sought to use nanoparticles, of sizes a thousand times smaller than a cancer cell, to deliver chemotherapy drugs specifically to the rogue cells themselves. This is done by exploiting some of the natural properties of tumours. Nanoparticles are injected into the bloodstream, attached with antibodies to recognise the cancer cell. Because aggressive cancer cells actively “eat” nanoparticles through the mechanism known as endocytosis, they end up self-assembling internal clusters of nanoparticles. This improves the toxicity problems of chemotherapy because large quantities of a drug can be delivered directly to the cancer without much harm to the surrounding healthy tissue. Gold nanoparticles are being used in this way in several ongoing clinical trials. However, even these therapeutic strategies still come up against the inevitable problem of cancers developing biological resistance to drugs.
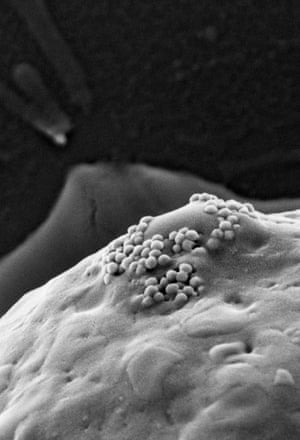
Instead, Lapotko’s idea has been to combine biology and physics in an entirely new way. Once gold nanoparticle clusters are inside a cancer cell, they are exposed to a short laser pulse which the nanoparticles convert to heat, forming a vapour bubble which expands and collapses in nanoseconds, called a ‘plasmonic nanobubble.’ The mechanical impact of this nanobubble tears the cancer cell apart in an instantaneous explosion.
“The nature of this explosion is intracellular so the surrounding healthy cells or important organs are not damaged,” Lapotko says. “A cell residue is left but this cannot reassemble into new cancer cells. It’s very safe as the energy of the laser pulse required is a million times lower than the laser energy used in some surgeries.”
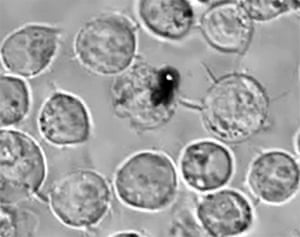
One of the common problems in cancer treatment is that when surgeons remove a tumour, they may leave residual tumours behind. “In many instances the cancer is in a part of the body where doctors are afraid to remove more than they think that have to,” says Masimo’s chief executive and founder Joe Kiani who is looking to bring Lapotko’s technology from academia to the clinic. “And when you leave some behind it metastasises. Recurrence and metastases are the main causes of death.”
But Lapotko’s technology can also be used to diagnose and eliminate before such remaining cells can grow into a far more dangerous and resistant recurrent tumour.
“We can administer nanoparticles one day prior to the surgery and after the surgeon removes the tumour, we apply the endoscope to the surgical bed,” Lapotko says. “If there are even single cancer cells left in the surgical margins, plasmonic nanobubbles are generated which produce a pressure pulse or acoustic pop which we can detect immediately in real-time with an ultrasound detector. And then we can use the mechanical impact of the same nanobubbles to destroy them.”
So far the technology has been tested on tumours in mice in a series of studies published by Nature Medicine and Nature Nanotechnology, with a dramatic improvement in survival rate and safety compared to existing treatments. The only limitation is for cancers deeper in the body where it is difficult to generate lethal plasmonic nanobubbles due to poor laser penetration into the deep tissue.
In these cases, Lapotko believes he can use the technology to improve the efficacy of the mainstream cancer therapy techniques. Radiotherapy works by disrupting the DNA helix in cancer cells, but by creating even small nanobubbles inside these cells beforehand, the DNA structure is already weakened, presensitising them so a far lower radiation dose can be administered to achieve the desired effect.

Lapotko is well aware of some of the disappointment among clinicians regarding nanomedicine after many years of promise, but still no broadly available treatments for patients. “There are two main reasons why not much has reached the clinic yet,” he says. “A lot of the time nanoparticles are initially developed for non-medical use, for example the energy industry or the oil industry and then people start thinking about medical applications. So perhaps they’re not so effective as they’re not initially designed with cancer in mind. And then within nanomedicine, the mainstream ideas aim to improve drugs, either by making nanoparticles which are drugs by themselves or making nanoparticles to carry drugs. So in cancer, nanomedicine did not replace chemotherapy, it has just created an additional chemotherapy, and because of that it faces the same regulatory challenges as any other drug.”
It typically takes 10-25 years and a lot of investment for anything to pass from academic research to drug use in the clinic, a passage referred to by scientists as the ‘Valley of Death.’ But with no pharmaceutical involved, Masimo are hoping to fast-track the process. They have obtained a grant from the National Institute of Health for further testing and intend to pursue phase I and II clinical trials within the next few years, likely to be held in Europe.
“A lot of the time what is done in the world of medicine on a mouse, doesn’t work on a monkey never mind a human but the early results look great,” Kiani says. “If it all works, we’re probably four years away from a product. But if it all works, it could be a game changer.”
Could a new approach to kill cancer at nanoscale work?
Hiç yorum yok:
Yorum Gönder