A trial of a new Alzheimer’s drug has shown it could benefit patients in the earliest stages of the disease, raising hopes that a treatment for the devastating condition may finally be on the horizon.
While the trial was designed to assess the safety of the treatment and not whether patients fared better on the drug, an “exploratory analysis” of the data revealed that the treatment appeared to slow the mental decline of patients who responded to the therapy.
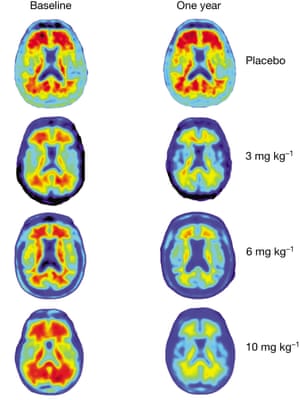
The small study of only 165 people with mild symptoms of the disorder found that a dozen monthly injections of the antibody aducanumab removed clumps of protein that build up in the Alzheimer’s brain.
A leading theory of the disease holds that the steady accumulation of a protein called amyloid-beta in the ageing brain kills off healthy neurons and brings about the memory and cognitive impairments experienced by Alzheimer’s patients.
In the trial, the strongest glimpse of mental improvement was seen in patients who had the highest dose of drug and who showed the greatest reduction in amyloid plaque proteins in follow-up brain scans. These patients did not worsen at all after six months of treatment. But the small number of patients enrolled in the study means that two much larger trials, which are now recruiting 2,700 patients in 20 countries, are needed to confirm whether the tantalising signs of benefit are real.
Alzheimer’s experts welcomed the results, but cautioned that it is too early to know whether the drug will be a help for patients. Other antibody treatments have looked impressive in early studies only to fail later on in larger trials.
John Hardy, a neuroscientist at UCL who first proposed that amyloid was a driver of Alzheimer’s disease, said: “It’s very interesting and nice to see all these positive data, and it has caused genuine excitement in the field, but it’s a very small number of patients and too small to draw any definitive conclusions from.”
The results from the trial led by the US biotech firm, Biogen, and a Swiss company called Neurimmune, are reported in the journal Nature. The data were first released at a scientific conference in March last year.
Aducanumab was hailed as a potential treatment for Alzheimer’s when scientists found the antibody in people who aged without suffering the sort of mental decline that goes hand in hand with old age. It appeared that the antibody prevented the build-up of amyloid plaques and staved off dementia.
When injected into Alzheimer’s patients, one or two in every thousand of the antibodies enter the brain where they latch on to wayward amyloid-beta proteins. Researchers at Biogen believe that other cells called microglia then arrive and clear the aberrant proteins from the brain. The drug appears to be most effective if the accumulation of amyloid protein is blocked before it causes too much damage. The process may start 15 years before people show symptoms.
In the latest trial, some patients experienced side effects. MRI scans showed a shift in the brain fluid that was more common at high doses and in people who carry the APOE type-4 gene, which is a major risk factor for Alzheimer’s disease. The scientists are now working on ways to avoid the side effect or diminish the problems by reducing the doses patients receive.
David Allsop, professor of neuroscience at Lancaster University, said the side effects will have to be overcome if the therapy is to find widespread clinical use. “Nevertheless, these findings could be a gamechanger if the effects on memory decline can be confirmed in more extensive follow-on studies.”
There are 850,000 people with dementia in Britain, a number that is expected to reach one million by 2025. Alzheimer’s is the most common form of the condition. “If this drug works, we’ll have a treatment for patients suffering from this devastating disease,” said Biogen’s Alfred Sandrock.
“These results provide tantalising evidence that a new class of drug to treat the disease may be on the horizon, said David Reynolds at Alzheimer’s Research UK.
James Pickett at the Alzheimer’s Society was similarly optimistic: “These results are the most detailed and promising that we’ve seen for a drug that aims to modify the underlying causes of Alzheimer’s disease.”
Trial shows tantalising signs that new Alzheimer"s drug could benefit early-stage patients
Hiç yorum yok:
Yorum Gönder